Understanding Energy
In this post from NET-ZERO:
How much energy does the sun shine on Earth? Why the sun provides enough energy to power the economy 7,000 times over.
How efficient are fusion, fission, and combustion reactions? Why E=mc2 tells us that the sun is 0.7% efficient, nuclear reactors are 0.1% efficient, and the combustion of fossil fuel is just 0.00000005% efficient.
What alternative energy options do we have? Burning coal, oil, and gas over the last 200 years has provided the surplus energy, knowledge, and innovation to create net-zero technologies for a sustainable future.
Fundamentals of Energy
Energy is the capacity for doing work.
It comes in many forms such as the gravitational potential of water in reservoirs, the kinetic energy of a spinning fly wheel, chemical energy stored in fossil fuels, thermal energy of vibrating molecules, or atomic energy used in nuclear power.
Energy can flow between different forms and can be put to work where needed for lighting, heating, mechanical movement, and in building the proteins which create life. The equation fundamental to understanding all energy is the most famous equation in modern science: E=MC2. Einstein showed us energy equals mass times the speed of light squared. Energy and mass are interchangeable: one can be converted to the other.
Fission Energy
The sun is a great example of this special relationship. A big nuclear reactor with large quantities of gas under extreme temperature and pressure. The sun squeezes hydrogen atoms together and through nuclear fusion creates helium.
The outgoing helium from the reaction weighs slightly less than the ingoing hydrogen. The lost mass is converted to pure energy and released into the universe as electromagnetic radiation. This is the very same radiation which warms the Earth and is essential to life on this planet. Every one-kilogram of hydrogen mass releases 180 million kWh of energy. If we were able to harness fusion reactions on Earth, it would take less than 1,000 tonnes of hydrogen to power the world each year.
The sun is also responsible for most of the energy we source here on Earth. The sun’s energy drives rainfall for hydroelectric dams and creates temperature differences which drives the wind for turbines. Sunlight is also the source of energy harnessed by plants over millions of years which formed the coal, oil, and gas we extract and burn.
The total power of sunlight reaching the planet’s surface is over 85 trillion kW which is 7,000 times more than the 12 billion kW used by humans to power everyday life. To radiate this amount of power across the universe, the sun consumes 600 million tonnes of hydrogen per second, creating the equivalent of 4 billion tonnes of light energy, an efficiency of 0.7%.
Sunlight hits the Earth’s surface with an average of 170 watt per square metre after reflective losses. The Earth’s surface area is 510,000 billion square metres so the total power reaching the planet is over 85,000 billion kW. Every second the sun consumes 600 million tonnes of hydrogen and transforms it into 596 million tonnes of helium and 4 million tonnes of light. That is 100 billion-billion kWh energy per second. Luckily, the sun is so large it will still shine for another 5 billion years.
Fusion Energy
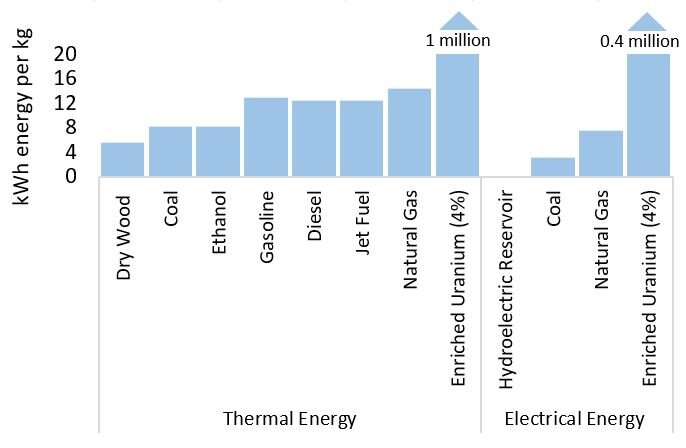
Nuclear reactors on Earth use fission rather than fusion. The reactions begin with uranium-235 which splits into smaller atoms of krypton and barium and in the process transforms 0.1% of the mass into energy. Fusion is seven times less efficient than the sun’s fission but still generates 22 million kWh of energy per kg of U-235. It would take less than 10,000 tonnes of pure uranium-235 to power the planet each year.
Combustion Energy
Burn fossil fuels and you are also taking advantage of Einstein’s E=MC2. During combustion, carbon atoms in gas, petrol, or coal, combine with oxygen to form carbon dioxide and 0.00000005% of the carbon mass is converted to energy, releasing 13 kWh per kg. This is 14 million times less efficient than fusion and is the reason we burn through 12 billion tonnes of fossil fuels each year.
Shifting Away from Fossil Fuels
Despite the low conversion efficiency, combustion does have other attributes in its favour. Fossil fuels have proven readily available, energy dense, easily transportable, and with a relatively easy route to release and harness the energy (combustion). Despite the low efficiency, fossil fuels supply 80% of energy today.
Whilst early humans relied upon sun, wind, and water for energy, the industrialised world today relies mostly on coal, oil, and gas. But as knowledge has exponentially increased, so has our ability to harness other energy sources more effectively.
The US army’s Manhattan Project, led by Robert Oppenheimer between 1939 and 1945, developed the first transportable atomic bombs used to devastating effect in Hiroshima and Nagasaki, Japan, in 1945. But advancements in the understanding of particle physics and nuclear fission had also helped Enrico Fermi and colleagues at the University of Chicago develop the first self-sustaining nuclear reactor in 1942.
During the same decade, Bell Laboratories demonstrated the first practical silicon solar cell designs which found their first commercial applications in outer space on the Vanguard 1 satellite in 1958.
The oil shortages of the 1970s were the driving force behind the development of modern wind turbines. The efficiencies of these technologies have steadily improved as costs have exponentially declined.
Today we have an abundance of competitive energy technology options. But the challenge of tomorrow is how to transition towards a more sustainable energy system.
Can we use the knowledge and technologies afforded us by burning fossil fuels over the last 200 years to transition to a net-zero future? In the book ‘Climate Change and the road to Net-Zero’ I argue that not only can we do it, but the whole energy system will end up 25% cheaper (than the fossil fuel equivalent) whilst also avoiding the worst impacts of climate change and air pollution.