Understanding the Greenhouse Effect
In this post from the book net-zero:
Who discovered global warming? The women’s rights campaigner who figured out the relationship between carbon dioxide and Earth’s temperature 150 years ago.
How will Planet Earth respond to increasing carbon dioxide? How scientists have correctly predicted the warming sensitivity of Planet Earth for over a century.
What is the greenhouse effect? One of those things that you learn at school but it’s actually way more complicated than your teachers made out (or maybe that was just me).
Discovering CO2 is the Atmospheric Thermostat
Eunice Newton Foote was an American scientist and women’s rights campaigner in the 19th century. She was a key signatory of the first Women’s rights convention in 1848 and 6 years later she become the first person to link atmospheric temperature with CO2.
By measuring the temperature rise of jars containing different gases, she predicted how carbon dioxide and water vapour concentrations could modulate solar heating:
“An atmosphere of that gas would give to our earth a high temperature; and if as some suppose, at one period of its history the air had mixed with it a larger proportion than at present, an increased temperature... must have necessarily resulted”. Eunice Newton Foote, “On the Heat in the Sun’s Rays”, 1856.
Her work was presented at the Annual Meeting for the American Association for the Advancement of Science in 1856 by a male colleague, Joseph Henry, but her results never made it into the conference proceedings. Her pioneering insights went unrecognised for 150 years until, in 2010, retired petroleum geologist Ray Sorenson came across her work in an 1857 volume of Annual Scientific Discovery.
Further laboratory work on CO2 and a range of other gases, published by Irish physicist John Tyndall in 1860, further supported Foote’s work. By 1890, Swedish chemist Svante Arrhenius had used the experimental data to make the first climate predictions. Arrhenius estimated that if the concentration of CO2 doubled from 280 to 560 ppm, temperatures would increase by 5⁰C.
At the time, though, his work was regarded as no more than scientific curiosity, nobody imagining that humans might actually change the atmosphere so drastically. Yet half a century later, a select few scientists started to take serious interest in Arrhenius’ work. In 1938, Guy Stewart Callendar presented the first evidence that Earth might already be warming. He used his own climate model to predict that, if the concentration of atmospheric CO2 doubled, the planet would be 2⁰C warmer.
The temperature increase due to concentrations of CO2 doubling in the atmosphere from pre-industrial times is now known as the climate sensitivity.
These early predictions have proven remarkably accurate, with CO2 concentrations now over 410ppm and temperatures already over 1⁰C higher. The best estimates from modern climate models, which use super computers and masses of data, suggest that doubling CO2 concentrations will lead to around 3⁰C total warming with the range of estimates between 1.5 and 4.5⁰C.
The Science Bit
The Green House Effect
Understanding the link between carbon dioxide (CO2) and temperature requires that we start with the all-important relationship between our planet and the sun.
Planet Earth is kept warm by the sun’s constant release of energy in the form of electromagnetic radiation. The most familiar and largest part of the electromagnetic spectrum is visible light with wavelengths between 0.4 and 0.7 micrometres (less than one thousandth of a millimetre) which our eyes use to see the world. Other parts of the spectrum include high energy, short wavelength UV light, and X-rays, alongside lower energy, longer wavelength infrared, microwaves, and radio waves. It’s this spectrum of the sun’s energy which warms your face on a summer’s day and which keeps the Earth temperate enough for life.
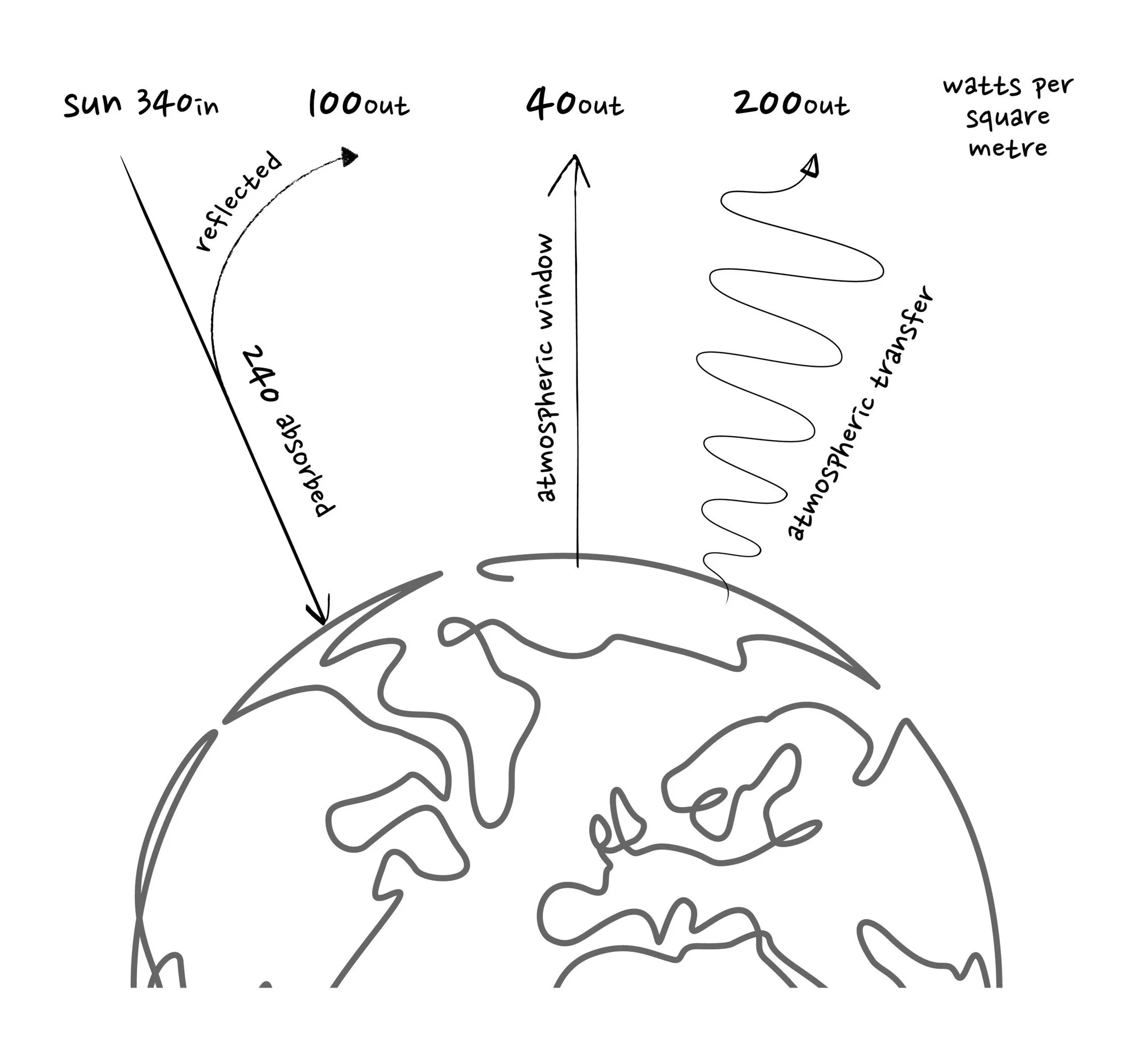
The sun radiates 380 trillion-trillion Watts (W) of power which reaches Earth’s orbit at 1,360 W per square metre. If you multiply this power by the area of the Earth’s shadow (130 trillion square metres), then a total of 173,000 billion kW of power constantly shines down on the planet. Divide the total power by the Earth’s surface area (510 trillion square metres) and an average of 340 W per square metre hits the planet at any one time.
Atmospheric gases, clouds, snow, ice, oceans, and the Earth’s surface reflect 30% (100 W per m2) of this incoming energy straight back into space and the remaining 240 W per square metre is absorbed by the atmosphere, land, and oceans.
The portion of the sun’s energy which is absorbed is re-radiated and sent back towards space as lower energy, longer wavelength, infrared radiation. Water vapour and greenhouse gases like CO2 in the atmosphere are more effective at absorbing this outgoing infrared and in turn re-radiate the energy in all directions. This serves to trap more heat before it escapes back into space, heating the lower atmosphere (troposphere), and cooling the upper atmosphere (stratosphere). This is the familiar phenomenon known as the “Greenhouse effect” coined by mathematician Joseph Fourier in 1824 before the process was fully understood.
The Extra Sciencey Bit
Radiative Physics, the Atmospheric Window, and the Lapse Rate
The physics of the greenhouse effect are a little (or maybe a lot) more nuanced than we are taught in school.
To better understand the phenomenon, first start by imagining that the Earth has no atmosphere. It simply heats up and re-radiates energy with nothing to slow the outgoing radiation, and all the energy is lost into space at the Earth’s surface.
The temperature of that surface can be calculated using the equation for a blackbody - the Stefan-Boltzman law - which says the rate of energy loss equals 5.67 x 10^(-8) multiplied by the temperature (in kelvin) to the power of 4.
We know 100 W per square metre of the sun’s power is reflected and 240 W per square metre is absorbed by Earth so, if the temperature is stable, 240 W per square metre must re-radiate back into space to balance the energy flows (or else the planet would continually heat up). Plugging 240 into the equation above tells us the temperature of the Earth would be 255 kelvin or -18⁰C with no atmosphere.
Luckily our planet isn’t floating through space completely naked. Earth is covered in a blanket of atmospheric gases like water vapour and CO2.
About 40 W per square metre of the energy re-radiated from Earth is emitted at a frequency which avoids being re-absorbed by atmospheric gases and escapes unhindered into space: this is known as the atmospheric window.
The remaining 200 W per square meter of outgoing energy is absorbed and re-radiated, bouncing around until it reaches a point high up in the atmosphere where greenhouse gases are few and far between and the radiation can finally escape to space.
This great escape happens about 5 km up in the atmosphere where gases like water vapour and CO2 become sparse. The temperature 5 km up is cooler than the surface of the Earth because gases of the atmosphere expand and become less dense at higher altitudes. Think about how cold it is at the top of a mountain or the temperature of an expanding aerosol spray on your skin. This temperature gradient from Earth’s surface and up through the atmosphere is called the lapse rate (it is also influenced by many other factors but that’s for another post).
Now imagine we instantaneously added an atmosphere to our naked, cold planet. Most of the energy is no longer lost straight from the surface but from the colder atmosphere where the lower temperature emits less radiation energy (thanks to the stefan-boltzman law). The lower radiation creates an energy imbalance, more energy is coming-in than going-out, and the planet and atmosphere heats up until the temperature at the average emission level reaches 255 kelvin again and the energy balance is restored.
So without the warming impact of greenhouse gases, the surface temperature of Earth would be -18⁰C and there would be no proliferation of complex life. Water vapour is the strongest infrared absorber and adds ~20⁰C to the Earth’s average temperature. Carbon dioxide, with the help of other greenhouse gases such as nitrous oxides, ozone, methane, and fluorocarbons, adds another ~13⁰C.
This brings the average surface temperature on Earth to 14-15⁰C which has proven optimal for modern humans and civilisation.
Now you can start to understand why adding even more CO2 to the existing atmosphere creates a heating effect. Firstly because CO2 is starting from a relatively low concentration in the air, the frequencies where the gas absorbs infrared are not saturated and can increase absorption in parts of the atmospheric window as concentrations increase. This prevents an extra portion of energy from escaping directly into space from the Earth’s surface. Instead, the energy is emitted from higher up in the colder atmosphere creating a small energy imbalance and an initial warming.
This initial temperature increase allows the atmosphere to hold more water vapour which further raises the height where the energy escapes to space. A higher emission level is colder and so even less radiation makes it to space until the whole system heats up enough to rebalance the energy flows - leaving the surface warmer.
Adding more greenhouse gases to the air partially closes the atmospheric window, creates a thicker blanket, and warms the planet.
Despite water vapour contributing a greater impact on warming overall, CO2 plays the most important role in controlling atmospheric temperature. This is because water vapour is self-regulating; if you add more water vapour to the atmosphere with no immediate change to temperature, the water vapour will condense back to liquid.
Greenhouse emissions are different because they remain in the atmosphere as a gas long enough to increase the temperature. Only then can the lower atmosphere hold more water vapour, which further amplifies the initial CO2 driven heating until no more water vapour can be accommodated.
Carbon dioxide acts as the planetary thermostat, whilst water vapour is the boost button.
Unless we find a way to limit the incoming solar radiation, drawdown greenhouse gases from the atmosphere, or transition to a net-zero carbon economy then the radiative physics, which has been understood for well over a century, tells us that the atmosphere and oceans will continue to heat up.
However, I believe we are now at a juncture where the range of possible values for the climate sensitivity, climate damages, and cost of net-zero technologies are sufficiently narrow to assert that a rapid transition to net-zero creates a win-win for all on Planet Earth.
For an elegant description of the physics of global warming check out this video by climate scientist Andrew Dessler.
For more details on the physics of Global Warming see Andrew Dessler’s ‘An Introduction to Modern Climate Change’ in the Bookshelf.